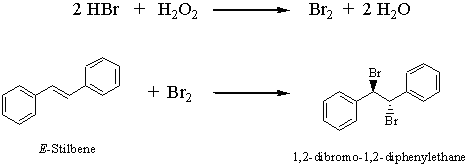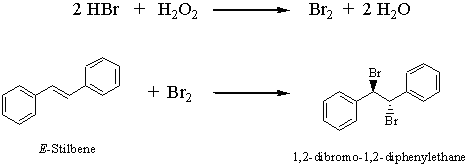A Greener Bromination of Stilbene
[adapted from Doxsee, K.M. and Hutchison, J.E. Green Organic Chemistry ]
Introduction
One of the first electrophilic addition reactions you learned in Chapter 7 was the bromination of an alkene using elemental bromine (Br2). It turns out that bromine is very dangerous to work with in the laboratory since it is volatile and highly corrosive, causing severe burns upon contact with skin. In this experiment you will generate Br2 in the reaction mixture (it is prepared in situ) , thus avoiding its hazards. You will brominate stilbene by using Br2 generated by oxidizing hydrobromic acid with hydrogen peroxide. The mechanism for the bromination is the same as the one you learned in lecture.
Experimental Procedure
SAFETY PRECAUTIONS: Hydrobromic acid is corrosive. Avoid contact and inhalation of vapors. Clean up any spills with a paper towel soaked with a little sodium bicarbonate solution. Hydrogen peroxide (30%) is a strong oxidizer and will damage your skin and clothing (the stuff you buy in the store is only 3%!).
Reaction
- Prepare a hot water bath using a shallow dish or beaker on a stirrer/hot plate.
- Place a magnetic stir bar, 0.5 g of E-stilbene, and 10 mL of ethanol in a 100-mL round-bottom flask. Fit the flask with a water cooled reflux condenser.
- Clamp the flask at the lowest joint so that it may be heated and stirred in the hot water bath. Stir while heating the mixture to reflux--the majority of the solid should dissolve.
- Slowly add 1.2 mL of concentrated 48% aqueous HBr. This addition will probably cause some of the stilbene to precipitate, but if you continue heating and stirring it should redissolve. Go on to the next step even if some solid is present.
- Measure out 0.8 mL of 30% hydrogen peroxide from the communal burette and add it dropwise to the reaction mixture. The initially colorless solution should change to a golden-yellow color.
- Continue to stir under reflux until the yellow color fades and the mixture becomes cloudy white (approximately 20 minutes).
Workup and Purification
- Remove the flask from the hot water bath and allow it to cool to room temperature. Carefully adjust the pH of the solution to pH 5 to 7 using concentrated aqueous sodium bicarbonate (NaHCO3) (you don't have to dip the pH paper into the mixture-just use your spatula to touch the pH paper with a little of the liquid from the mixture). It may take very little NaHCO3 to neutralize the acid.
- Cool the mixture further using an ice bath. Isolate the product by vacuum filtration.
Characterization
- Determine the mass and melting point of the product (lit value mp = 241 oC) and the NMR.